There are two main choices when it comes to cylinders these days: 232 or 300 bar.
300 bar cylinders are heavier, but hold significantly more gas than a similarly-sized 232. That is the argument for them.
The main arguments against them are: The number of people who complain they never get full 300 bar fills, and the Real Gas Laws.
When we learn to dive, we are taught that the calculate the amount of gas in a cylinder, we multiply the pressure by the volume. Therefore a 232 bar 10 litre cylinder holds 2320 litres of air.
Sadly, this is not actually true. This would be the case for an Ideal Gas, which would always go up in a linear way: Every doubling of the amount of gas in a cylinder would double the pressure. At low pressures, air is close enough to an ideal gas for the maths to work.
Once past the low 200s tho, air becomes very non-Ideal. A 10 litre cylinder charged to 300 bar does NOT hold 3000 litres of air. This is because the pressure increase is not made up solely of the increase in air molecules, it is also made up of repulsion between the molecules - Vaan der Waals forces.
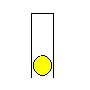
The simplest way to understand this concept is: Go to a sports shop and buy a can of tennis balls - one of those metal cans that holds three tennis balls. Now, take the balls out, and hold the can upside down.
Put a single tennis ball in, and hold it there with your hand. Your hand is working aginst a force equal to the weight of a single tennis ball.

Put in a second tennis ball. Your hand is now working aginst a force equal to the weight of two tennis balls.
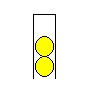
Now add the third. Of course, your hand is working aginst a force equal to the weight of THREE tennis ball.
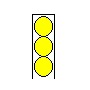
Now, put a fourth ball into the tin. So that it's completely inside the tin.
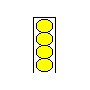
Not easy, is it? You've got to really push to get four balls into a space big enough for three. Even though you've only added one more tennis ball, you've greatly increased the amount of effort it takes to hold those four balls in place.
This is exactly what happens to a cylinder full of gas, only a lot more than four molecules are involved. When you first add the molecules, they're far enough apart to not interfere much with each other. You get a linear relationship between amount of gas, and pressure.
Eventually, the molecules get close enough to start pushing against each other, and pressure goes up for a different reason.
Or, if you want a really, really simple way to understand it: Above a certain pressure, air starts to act like a liquid, and liquid are non-compressible.
What is the practical outcome of this?
When it comes to normal air, it just means that you aren't carrying as much air as you might think you are. That's all. But when you start down the Nitrox road...
Let's imagine you want to fill a 300 bar cylinder to be 50% Oxygen and 50% Nitrogen. And to make the maths easy, let's assume you're not using air - you're using a bottle of Oxygen and a bottle of Nitrogen.
If you add 150 bar of Oxygen, and then top it up with Nitrogen until it reaches 300 bar, will you have a 50-50 mix?
Nope. Because whilst 150 bar in a 10 litre cylinder means 1500 litres of air, 150 to 300 bar of Nitrogen is NOT made up of 1500 litres of Nitrogen. Because neither Oxygen nor Nitrogen is an Ideal Gas. Therefore, there will be more Oxygen than Nitrogen.
This inherent difficulty of getting an exact percentage of mixed gases with a high-pressure cylinder makes 232 bar cylinders the choice of most mixed-gas divers.
For a much more technical explanation, you need NigelH's page on Van der Waals